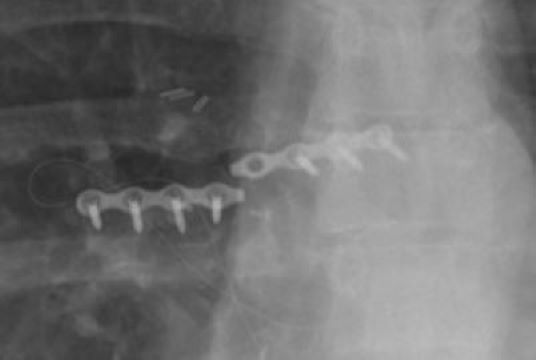
Fractured Titanium "Rib Plates"
Broken ribs and sternum bones are injuries which surgeons can treat by implanting titanium “rib plates” – medical devices sold by KLS Martin, LP, and other manufacturers – to hold the broken bones together while they heal. These are typically semi-flexible metal strips with centerline holes through which bone screws are inserted to fasten the device to the underlying bone. Typically, the “rib plate” is placed lengthwise down the broken rib with its mid-point over the fracture. Bone screws are inserted through the centerline holes of the “rib plate” on both sides of the fracture.
Unfortunately, there are instances where bone healing occurs but the “rib plate” fractures. Simple x-ray testing can diagnose a fractured “rib plate.” An actual fractured “rib plate” is shown below.
Problems Attributed to Fractured Titanium Rib Plates
Scientific studies have shown that KLS Martin titanium rib plates can fatigue and then fracture when subjected to repeated frequent bending movements caused by ordinary activities like breathing.
When “rib plates” fracture, they can cause great pain and discomfort which persists until the broken device is surgically removed. Such corrective surgeries are, of course, a new medical wound. Persons with fractured rib plates may sustain a wide range of damages including severe physical pain and suffering, medical costs and expenses, loss of income, and hospitalization for corrective surgery.
Holding Medical Device Manufacturers Accountable
The public relies on medical device manufacturers to thoroughly test their products and warn treating physicians of their risks and potential harm. If treating physicians (called “learned intermediaries”) are not provided with an adequate warning of this fatigue-induced fracture process, then they cannot fully consider whether the use of this medical device is appropriate for the medical needs of their patients. Similarly, the patient cannot give fully informed consent to the implant of the rib plates into their bodies.
If you have had surgery to repair broken ribs and now have a fractured “rib plate” that is causing pain and discomfort, you should immediately contact Lamothe Law Firm. We can help you with the process of determining your recovery rights.
If your surgeon has advised you to have the broken “rib plate” surgically removed, it is essential that your surgeon and/or your hospital’s pathology department be immediately notified to preserve the broken device. Lamothe Law Firm can assist you.
Contact an Experienced Medical Product Liability Attorney
Proving that a medical device is defective can be complicated, requiring thorough investigation, consultation with biomechanical engineers and other experts, and taking on large corporations and their insurance companies. There also are time limits for filing suit and circumstances where a rib plate seller might have defenses to a claim. Lamothe Law Firm, LLC can assist you in determining the status of your claim.
If you or someone you love is the victim of a defective titanium “rib plate,” whether a KLS Martin, LP product or one manufactured by another company, you need experienced and courtroom-tested trial lawyers on your side. The Attorneys at Lamothe Law Firm, LLP, have represented persons affected by fractured “rib plates” and other defective medical devices. Please contact us today for a free consultation to learn how we will fight for you.